Abstract
Background: Aggressive B-cell non-Hodgkin lymphomas (NHL) are a diverse group of neoplasms, with diffuse large B-cell lymphoma (DLBCL) being the most common form. While the majority of patients are cured with first-line chemoimmunotherapy, the management of relapsed/refractory (R/R) disease can be challenging. Many patients with R/R DLBCL after first-line therapy are unsuitable for aggressive salvage chemotherapy, autologous stem cell transplantation (ASCT), and/or chimeric antigen receptor T-cell therapy. Many others do not respond to aggressive salvage chemotherapy or achieve a suboptimal response and do not proceed to curative intent therapy, and this usually results in a poor prognosis. Mosunetuzumab is an off-the-shelf CD20xCD3 T-cell engaging bispecific monoclonal antibody that redirects T cells to eliminate B cells and is administered as a fixed-duration regimen with Cycle 1 step-up dosing to mitigate the risk of severe cytokine release syndrome (CRS). In a Phase I trial, mosunetuzumab showed promising single-agent efficacy and a manageable safety profile in patients with B-cell NHL, supporting the clinical development of combination regimens (Budde et al. J Clin Oncol 2022). Polatuzumab vedotin is a CD79b targeted antibody-drug conjugate that delivers a microtubule-disrupting agent, monomethyl auristatin E, directly to B cells. Mosunetuzumab in combination with polatuzumab vedotin (M+Pola) has shown synergistic anti-lymphoma activity in a preclinical model and promising efficacy and safety in a Phase Ib/II trial, with high response rates (overall response rate: 66%; complete response rate: 49%) and a low rate of CRS events (all low grade) in R/R aggressive NHL (Budde et al. ASH 2021).
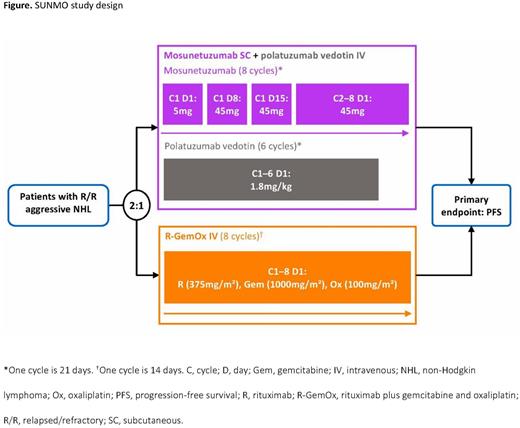
Methods: SUNMO (NCT05171647) is a randomized, open-label, multicenter, Phase III trial that will evaluate the efficacy and safety of treatment with subcutaneous mosunetuzumab + intravenous (IV) polatuzumab vedotin in patients with R/R aggressive B-cell NHL in comparison with a standard of care regimen in this patient population: rituximab, gemcitabine and oxaliplatin (R-GemOx, IV). Patients with CD20-positive R/R aggressive NHL (DLBCL not otherwise specified [NOS], high-grade B-cell lymphoma NOS or double/triple hit, transformed follicular lymphoma, and Grade 3B follicular lymphoma), who have received at least one prior systemic therapy (patients who have received only one prior line of therapy must be ineligible for ASCT) and have an Eastern Cooperative Oncology Group (ECOG) performance status 0-2, are eligible for this trial. Exclusion criteria include prior treatment with mosunetuzumab or other CD20-directed bispecific antibodies, polatuzumab vedotin, R-GemOx, or GemOx; allogeneic stem cell transplantation; and current or past history of central nervous system involvement of lymphoma. Eligible patients will be randomized 2:1 to receive either M+Pola or R-GemOx, stratified by the number of prior lines of therapy (1 vs ≥2) and response to last therapy (relapsed vs refractory). Patients will receive treatment for a fixed duration of eight cycles (six cycles for polatuzumab vedotin), unless progressive disease or unacceptable toxicity occurs earlier (Figure). The primary endpoint is progression-free survival (PFS) determined by an independent review facility (IRF). Treatment comparisons will be made with a 2-sided level 0.05 stratified log-rank test. Secondary endpoints include overall survival; IRF- and investigator-assessed complete response rate, objective response rate, duration of response, and duration of complete response; PFS (investigator-assessed); patient-reported outcomes; and safety. Biomarker analyses include a pre-specified analysis of prognostic subsets from baseline biopsies and circulating tumor DNA at baseline and during treatment. Enrollment began on April 26, 2022, and an estimated 75 sites globally will participate to enroll approximately 222 patients (M+Pola: 148 patients; R-GemOx: 74 patients).
Disclosures
Westin:ADC Therapeutics: Consultancy, Research Funding; Kite, a Gilead Company: Consultancy, Research Funding; Genentech/Roche: Consultancy, Research Funding; AstraZeneca: Consultancy, Research Funding; MorphoSys/Incyte Corporation: Consultancy, Research Funding; Iksuda: Consultancy; MonteRosa: Consultancy; Merck: Consultancy; Abbvie/GenMab: Consultancy; Calithera: Consultancy, Research Funding; Bristol Myers Squibb: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; SeaGen: Consultancy. Kim:Beigene: Research Funding; Takeda: Honoraria; Sanofi: Research Funding; Kyowa-kirin: Research Funding; Boryong: Research Funding; Donga: Research Funding. Wu:Amgen: Ended employment in the past 24 months; F. Hoffmann La Roche, Ltd.: Current Employment. Yin:F. Hoffmann La Roche, Ltd.: Current equity holder in publicly-traded company, Divested equity in a private or publicly-traded company in the past 24 months; Genentech, Inc.: Current Employment, Patents & Royalties. Pham:F. Hoffmann La Roche, Ltd.: Current Employment. Penuel:Genentech, Inc.: Current Employment, Current equity holder in publicly-traded company. Jing:Genentech, Inc.: Current Employment, Current holder of stock options in a privately-held company. Wei:F. Hoffmann La Roche, Ltd.: Current equity holder in private company, Current holder of stock options in a privately-held company, Patents & Royalties; Genentech, Inc.: Current Employment. Budde:Genentech: Other: Advisory Board; ADC Therapeutics: Other: Advisory Board; Merck: Research Funding; AstraZeneca: Research Funding; Amgen Inc: Research Funding; Ziopharm Oncology Inc: Other: DMSC member for a phase 1 clinical trial.
OffLabel Disclosure:
Mosunetuzumab is a CD20xCD3 bispecific antibody that redirects T cells to engage and eliminate malignant B cells. Mosunetuzumab is an investigational agent in the United States. Polatuzumab vedotin (Polivy) is a CD79b-directed antibody-drug conjugate indicated in combination with bendamustine and a rituximab product for the treatment of adult patients with relapsed or refractory DLBCL, not otherwise specified, after at least two prior therapies.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal